Coacervation is a process that protects and delivers synbiotics - combinations of probiotics and prebiotics - by forming a protective coating around beneficial microorganisms. This method ensures they survive harsh digestive conditions and reach the right spot in your digestive system.
Key Takeaways:
- Why It Matters: Encapsulation improves the survival of probiotics, ensuring they remain effective during digestion.
- How It Works: Coacervation uses liquid-liquid phase separation to form a shell around probiotics, protecting them from stomach acid and enzymes.
-
Techniques:
- Simple coacervation (single polymer) or complex coacervation (oppositely charged polymers) enhance stability.
- Specialized lipid coatings, like those in Begin Rebirth RE-1™, deliver high CFU counts without refrigeration.
- Challenges: Scaling production, maintaining particle size, and extending shelf life are ongoing hurdles.
This technique is crucial for creating effective synbiotic products, ensuring probiotics and prebiotics work together to support gut health.
Core Coacervation Methods
Types of Coacervation
Synbiotic encapsulation can use either simple coacervation (a single polymer system) or complex coacervation (a combination of oppositely charged polymers to create durable shells). Complex coacervation often provides better stability and protection against stressors like heat, moisture, and pH changes. It also allows for controlled size and thickness of the shell. These differences highlight key process parameters, which are outlined below.
Process Control Factors
Several factors play a crucial role in ensuring the success of coacervation:
- pH range: 4.0–4.5
- Temperature: 68–77°F (20–25°C)
- Ionic strength: Balanced salt levels
- Polymer concentration: Determines shell thickness
Even small deviations - for example, a ±2°F change in temperature or ±0.2 pH units - can disrupt uniform coating formation. Maintaining these parameters is essential for a successful encapsulation process.
Process Steps
-
Dispersion Phase
Synbiotics are dispersed in a carrier solution under strict mixing and temperature controls. -
Polymer Addition
Coating materials are added carefully. For complex coacervation, oppositely charged polymers are introduced one after the other to form the protective shell. -
Shell Formation
Environmental conditions, such as pH and temperature, are finely adjusted to initiate coacervation. These adjustments help create a uniform and consistent coating. -
Hardening
The final step involves stabilizing the capsules, either through controlled cooling or chemical cross-linking, to ensure durability.
Each step requires precise control to protect the synbiotics effectively and ensure the success of the coacervation process.
Materials Used in Synbiotic Coacervation
Polymer Types
Choosing the right polymers is key to effective synbiotic encapsulation. Commonly used options include:
- Natural Proteins: Gelatin, whey proteins, casein derivatives
- Polysaccharides: Alginate, chitosan, pectin, carrageenan
These materials are popular because they work well with biological systems and create stable coacervates. Combining proteins and polysaccharides often boosts protective capabilities. Beyond the polymer itself, factors like material properties and safety are essential for successful encapsulation.
Material Requirements
Encapsulation materials need to shield microorganisms from harsh gastrointestinal conditions, such as acids, enzymes, and bile salts. At the same time, they should enable controlled release at the right location in the body. These materials must also meet safety standards and be produced with consistent quality.
Synbiotic Material Interactions
The way encapsulation materials interact significantly impacts how well the system performs. A solid encapsulation strategy ensures probiotics survive tough digestive environments and stay effective until they reach their target. For example, advanced delivery systems like Lyosublime™ can deliver up to 500 billion CFU per serving, along with about 4.5 g of prebiotic fiber, ensuring rapid absorption [1]. Other important factors include maintaining capsule stability during storage, releasing contents at the right time and place, and compatibility with prebiotic components. These interactions are usually assessed through tests for stability and bioavailability.
Testing Coacervate-Encapsulated Synbiotics
Evaluating Release and Absorption
To understand how these encapsulated synbiotics perform, researchers simulate gastrointestinal (GI) conditions in the lab. Using in vitro models, they measure how and when the synbiotics are released, ensuring they remain active throughout the process. These tests also help determine how well the body can absorb the synbiotics, confirming their effectiveness.
Verifying Delivery Efficiency
Delivery efficiency is crucial - synbiotics must reach specific areas of the GI tract to work as intended. Researchers use imaging and tracking technologies to observe how the components are distributed. These tests fine-tune the encapsulation process and ensure the synbiotics deliver the intended health benefits.
sbb-itb-1bbfe7f
Limitations and Future Development
Current Challenges
Scaling up production comes with its own set of hurdles, including temperature changes, inconsistent particle sizes, short storage life, and high manufacturing costs. Even small temperature shifts or uneven particle sizes can reduce encapsulation efficiency during large-scale production. Additionally, the limited shelf life of current formulations at room temperature poses a barrier to making these products commercially viable.
New Technologies
Efforts are underway to tackle these obstacles with new approaches. For example, advanced polymer systems are being designed to improve temperature stability and provide consistent encapsulation under a wider range of conditions. Automated process control systems are also being integrated into manufacturing to reduce variability in particle size, resulting in more precise production.
Improving Results
The focus now is on refining outcomes by combining cutting-edge methods. Hybrid techniques, such as pairing coacervation with modified atmosphere packaging, are showing potential for extending shelf life and boosting stability. Researchers are also exploring ways to optimize cross-linking processes using natural compounds and testing protective additives like specialized antioxidants to strengthen encapsulated synbiotics and improve their durability. Strict quality control measures, including detailed particle size analysis and stability testing, are being implemented to ensure reliable and consistent results.
Microencapsulation Technology for Health Ingredients
Summary
Coacervation plays a key role in protecting synbiotics, maintaining their stability during storage and gastrointestinal transit. This method ensures precise control over particle formation, helping keep microorganisms alive and effective.
Advanced delivery methods, such as Lyosublime™ in Begin Rebirth RE-1™, take encapsulation to the next level by improving absorption speed and enhancing results for users [1]. Further developments in polymer systems and process controls are expected to boost stability and consistency even more [1].
Independent studies reveal noticeable benefits, with many participants reporting reduced bloating and fewer allergy symptoms [1]. These findings demonstrate how proper encapsulation can maximize the effectiveness of synbiotics.
Researchers continue to work on making encapsulation even more efficient, focusing on better protection for probiotics and precise release timing. These efforts pave the way for cutting-edge products like Begin Rebirth RE-1™.
Begin Rebirth RE-1™
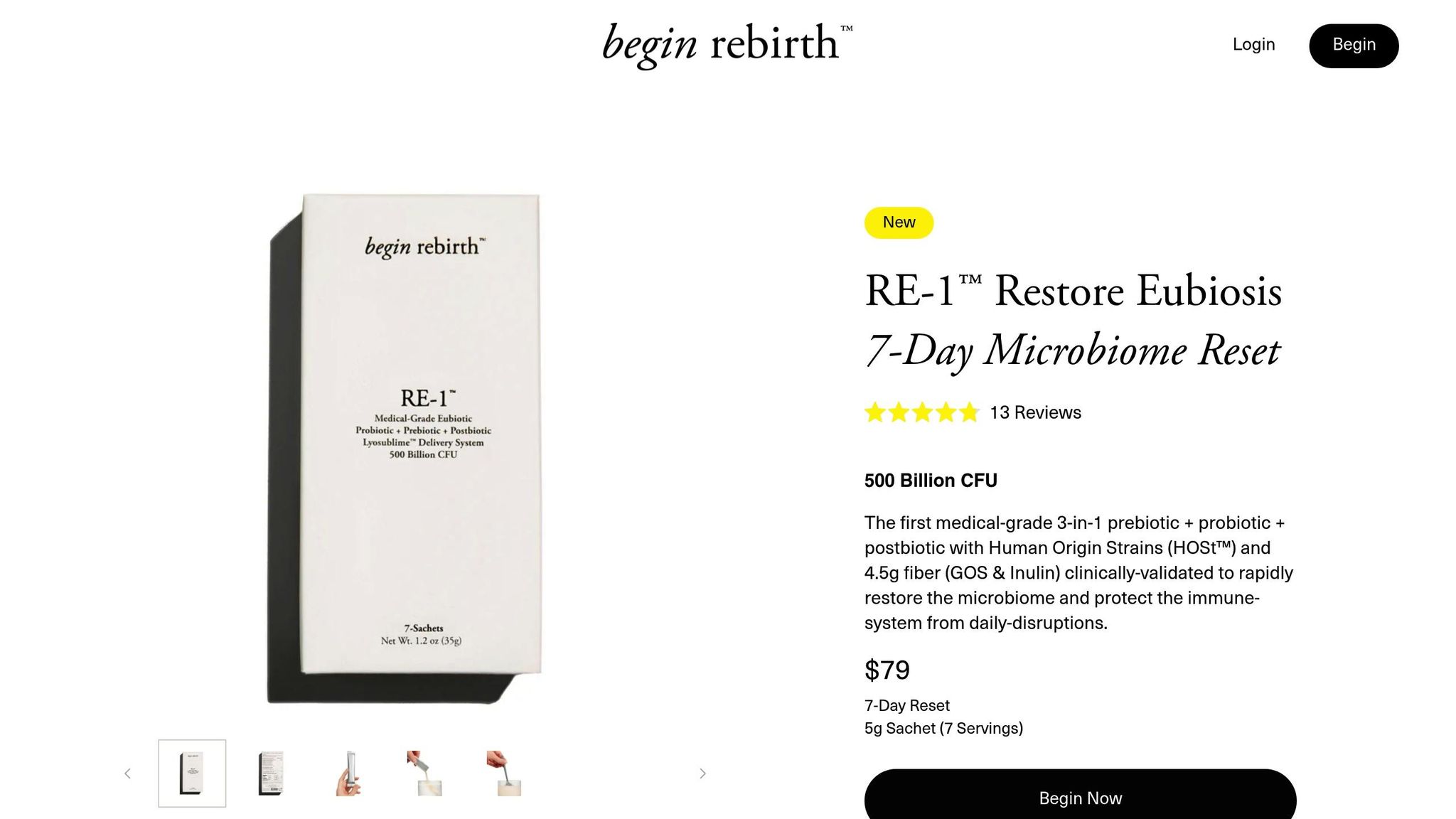
Begin Rebirth RE-1™ uses a specialized Lyosublime™ delivery system to protect and deliver its active ingredients effectively. This medical-grade synbiotic combines 7 live probiotic Human Origin Strains (HOSt™), 2 fiber-based prebiotics, and 1 immune-supporting postbiotic to help balance the microbiome and support immune health. The ingredients are carefully designed to work together, ensuring the active strains are delivered where they’re needed most. Impressively, 7 out of the 9 strains are lipid-microencapsulated, making them resistant to stomach acids, digestive enzymes, and bile salts. Thanks to the Lyosublime™ system, absorption is optimized throughout the digestive system.
Here’s a quick breakdown of the key components and their delivery benefits:
| Component | Delivery Mechanism | Benefits |
|---|---|---|
| HOSt™ Strains | Lipid Microencapsulation | Improved survival in acidic environments |
| Prebiotics (GOS & Inulin) | Lyosublime™ Delivery System | Better absorption and stability |
Clinical validation backs up these claims. A third-party study found that 94% of participants reported reduced bloating and abdominal discomfort within just 7 days [1]. Manufactured in Japan under strict quality controls, this product ensures consistent, reliable results.