Th17 cells are critical for immune defense, but their balance is key - too much activity can lead to inflammation, while too little weakens barriers. Probiotics help regulate these cells by influencing gut bacteria, cytokines, and immune signaling pathways. Here's what you need to know:
- Th17 Cells: Protect mucosal surfaces by producing cytokines like IL-17A (fights infections) and IL-22 (maintains barriers). Imbalance can cause autoimmune issues like IBD.
- Probiotics' Role: Probiotics adjust gut bacteria, boost short-chain fatty acids, and influence cytokines (IL-23, IL-6, TGF-β) that guide Th17 development.
-
Strain-Specific Effects:
- Lactobacillus: Promotes regulatory T cells.
- Bifidobacterium: Reduces inflammation.
- Bacillus: Enhances mucosal immunity.
- Probiotic Mechanisms: Act via Toll-Like Receptors (TLRs) to trigger immune responses and regulate transcription factors like RORγt and STAT3.
These findings suggest probiotics could help manage inflammatory diseases by balancing Th17 activity. Products like Begin Rebirth RE-1™ show promise but need more research for tailored therapies.
Th17 Cell Development and Function
Signals That Drive Th17 Formation
Th17 cells develop through specific molecular signals that direct naive T cells toward the Th17 lineage. When dendritic cells encounter pathogens, they release cytokines like IL-23, IL-6, and TGF-β. These signals activate the transcription factor RORγt, which is key to Th17 differentiation.
The gut environment plays a big role in this process. Short-chain fatty acids (SCFAs) produced by gut microbes influence Th17 cell development and help regulate immune responses.
Segmented filamentous bacteria (SFB) also play a part. These bacteria attach to intestinal epithelial cells, triggering local reactions that promote Th17 formation. These mechanisms not only shape Th17 development but also affect their role in immunity and disease, highlighting their dual nature in health and pathology.
Th17 Cells in Health and Disease
Th17 cells are essential for maintaining balance between protection and inflammation at mucosal sites like the gut and lungs. They produce several key cytokines:
| Cytokine | Primary Function | Impact on Health |
|---|---|---|
| IL-17A | Antimicrobial defense | Fights fungal and bacterial infections |
| IL-22 | Barrier maintenance | Strengthens epithelial defenses |
| IL-21 | Immune regulation | Coordinates T and B cell activities |
While Th17 cells are vital for defense, excessive activity can lead to chronic inflammation. In autoimmune diseases, overactive Th17 responses may cause tissue damage, making these cells a focus for potential therapies.
In the gut, balanced Th17 activity is crucial. They maintain intestinal barrier health and prevent harmful microbes from overgrowing. Disruptions in this balance have been linked to conditions like inflammatory bowel disease (IBD) and celiac disease.
Ongoing research into the pathways that regulate Th17 cells is opening doors to new treatments. By targeting the processes involved in their development, scientists aim to create better therapies for autoimmune disorders and infections.
Th17 Cells: Development, Differentiation and Function
How Probiotics Change Th17 Cell Behavior
Cytokine signals play a key role in Th17 cell differentiation. Let’s dive into how probiotics influence these pathways directly.
How Probiotics Affect Cell Development
Probiotics influence Th17 cell development by interacting with intestinal epithelial and dendritic cells. They stimulate Pattern Recognition Receptors (PRRs) and shift cytokine production, particularly IL-23 and IL-6, which helps shape the immune environment in the gut.
Microbiome Adjustments and Immune Impact
When probiotics colonize the gut, they alter the microbiome's composition, which can directly influence Th17 cell behavior. By competing with harmful bacteria for resources and attachment sites, probiotics help maintain a balanced microbial ecosystem. Additionally, probiotic metabolites like short-chain fatty acids (SCFAs) adjust the local pH, strengthen the gut barrier, and regulate inflammatory gene activity. These effects depend on the specific probiotic strain, as detailed below.
Strain-Specific Effects on T-Cell Balance
Each probiotic strain interacts differently with the immune system, tailoring cytokine responses to fine-tune immune balance. Here's a breakdown of their specific effects:
| Probiotic Type | Primary Effect on T-Cells | Impact on Immune Balance |
|---|---|---|
| Lactobacillus species | Regulates Th17 development | Encourages regulatory T cell growth |
| Bifidobacterium strains | Lowers pro-inflammatory signals | Promotes balanced immune responses |
| Bacillus species | Adjusts cytokine production | Supports mucosal immunity |
The timing and duration of probiotic use are crucial. Consistent intake helps maintain healthy Th17 levels, avoiding excessive inflammation that could harm tissues.
These insights open doors to designing probiotics aimed at balancing immune responses, rather than fully suppressing or over-activating Th17 cells.
sbb-itb-1bbfe7f
Cell Signaling in Probiotic-Th17 Interaction
This section delves into the molecular signaling mechanisms behind how probiotics influence Th17 cell development.
TLR Pathways and Immune Response
The immune system detects probiotics through Toll-Like Receptors (TLRs), which recognize Microbe-Associated Molecular Patterns (MAMPs). This detection triggers signaling pathways that shape immune reactions.
Here’s how different TLRs respond to probiotic components:
| TLR Type | Probiotic Component | Immune Response |
|---|---|---|
| TLR2 | Peptidoglycan | Activates dendritic cells |
| TLR4 | Lipopolysaccharides | Triggers cytokine production |
| TLR9 | Bacterial DNA | Stimulates interferon release |
These TLR-activated signals influence transcription factors that are key to Th17 cell differentiation.
Regulation of Key Transcription Factors
Probiotics play a role in shaping Th17 cell development by affecting critical transcription factors. Two major players are:
- RORγt: The main regulator driving Th17 differentiation.
- STAT3: A signal transducer activated by cytokines like IL-23 and IL-6.
These factors control the gene expression needed to guide naive T cells into the Th17 lineage. Certain probiotic metabolites can either boost or suppress their activity, allowing for precise immune response adjustments.
The transcriptional shifts caused by probiotics also influence the local cytokine environment, further regulating immune function.
Impact on Immune Signaling Molecules
Probiotic bacteria modify the production and balance of essential immune signaling molecules, including:
- Interleukin-23 (IL-23): Supports the survival and function of Th17 cells.
- Interleukin-6 (IL-6): Initiates the development of Th17 cells.
- TGF-β: Works alongside IL-6 to promote Th17 differentiation.
The concentration and timing of these signals determine whether the immune response leans toward inflammation or regulation. Probiotic metabolites can directly affect cytokine production in intestinal cells, helping to maintain a balanced immune environment.
These molecular insights explain why different probiotic strains can have varying effects on the immune system. Such understanding is crucial for designing targeted probiotic therapies for immune-related disorders.
Medical Applications and Research Status
Treatment Options for Inflammatory Diseases
Recent studies suggest that advanced probiotic formulations could play a role in regulating immune responses linked to Th17 cell activity. Early findings hint at their ability to help restore immune balance, but more research is needed to confirm these effects. One example is Begin Rebirth RE-1™, which incorporates specialized probiotic technology designed to influence immune function.
Begin Rebirth RE-1™ Features
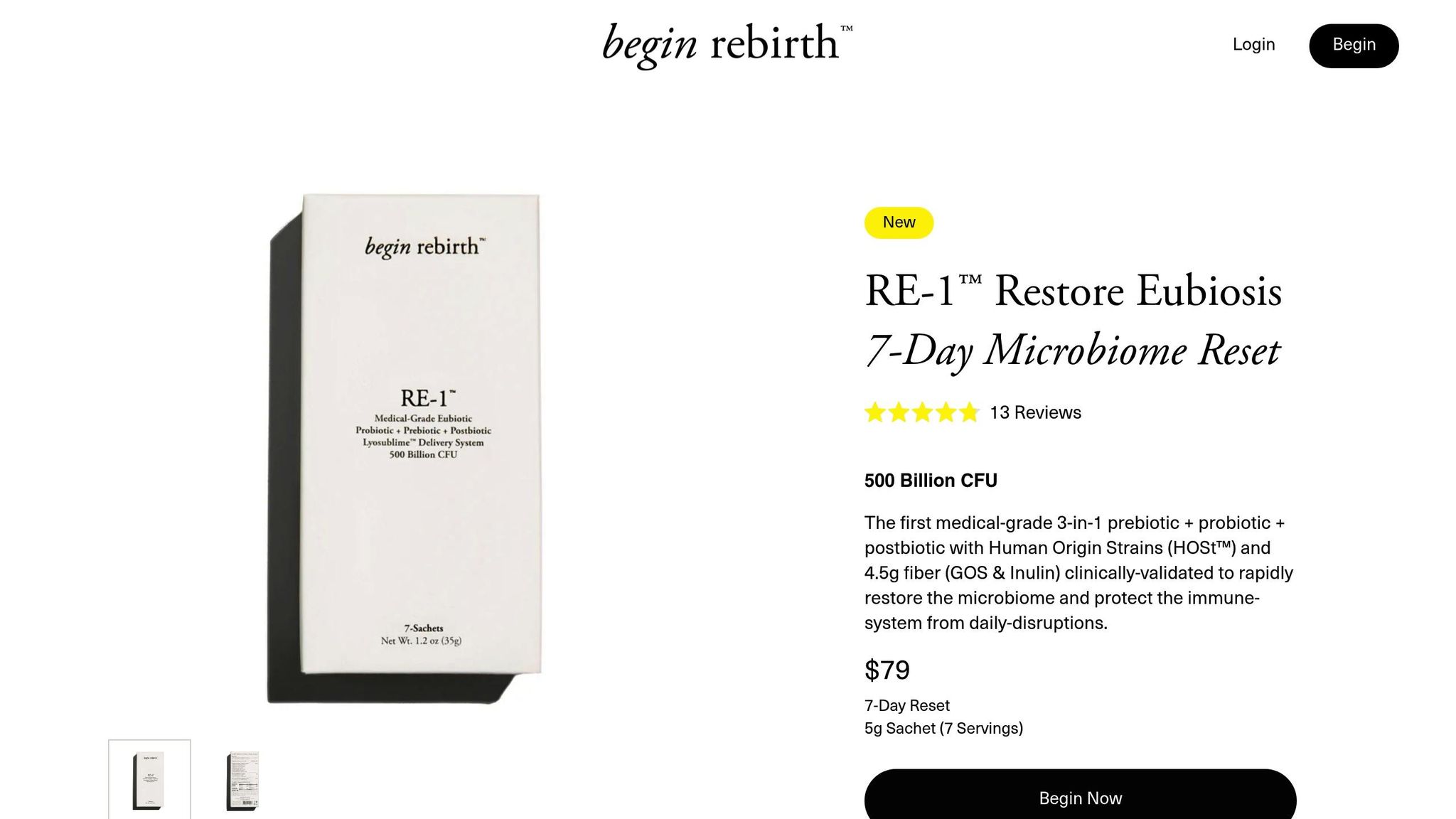
Begin Rebirth RE-1™ is a medical-grade supplement combining probiotics, prebiotics, and postbiotics in a single formulation. Its key components include:
- 500 billion CFU per serving with HOSt™ strains
- 4.5g of prebiotic fiber (GOS and Inulin)
- Lyosublime™ delivery system for improved absorption
Preliminary data highlights its potential benefits. For instance, RE-1™ has been shown to ease gastrointestinal discomfort and lower allergic reactions [1].
An observational study involving 35 adults aged 20–65 revealed that 80% of urban participants lacked Human Origin Strains (HOSt™) [1]. This finding emphasizes the potential value of targeted probiotic solutions like RE-1™.
While these advancements in probiotic therapy are encouraging, further research is essential to better understand the mechanisms involved and refine treatment strategies.
Summary and Future Directions
Key Takeaways
Probiotic strains impact Th17 cell development through:
- Direct effects on immune signaling
- Changes in gut microbiome composition
- Regulation of transcription factors
These mechanisms, as outlined earlier, highlight potential therapeutic applications for managing inflammatory conditions. Advanced probiotic combinations - integrating prebiotics, probiotics, and postbiotics - show how multiple pathways can influence immune responses. These findings open doors for more focused research to address unanswered questions.
Future Research Priorities
To build on these findings, upcoming studies should aim to:
- Investigate how specific strains affect Th17 cells.
- Identify detailed signaling pathways, cellular receptors, and determine ideal dosing strategies.
- Assess long-term use, combination treatments, and how different patients respond.
Products like Begin Rebirth RE-1™ offer encouraging results, but further research is needed to refine these formulations for targeted immune conditions and diverse patient needs.